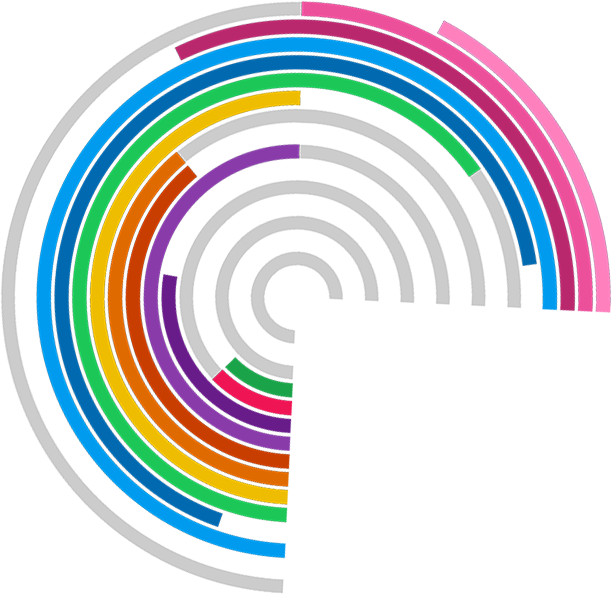-
 A systematic approach tomaking trials more efficientThe evidence base for how to make the trials process
A systematic approach tomaking trials more efficientThe evidence base for how to make the trials process
efficient is remarkably thin. Trial Forge aims to change this.Explore Pathway Learn More
Trials
Randomised controlled trials are the gold standard for evaluating healthcare treatments; 1000s are done every year.
Essential
Randomised trials are the cornerstone of evidence-based healthcare because they offer the fairest tests of treatments, therapies and initiatives.
Inefficient
The evidence base for how to make the trials process efficient is remarkably thin. Trial Forge aims to change this.
Small Changes add up
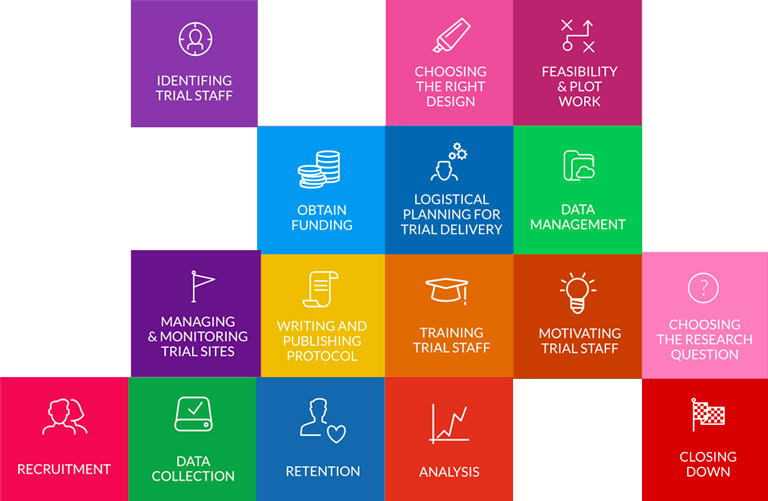
Large, single improvements are nice to have but rather rare. Marginal gains – small improvements to processes – start to add up if look across a whole system. Trial Forge aims to look across all trial processes with the intention of trying to improve them all, even if it’s just by a tiny amount, because these gains will start to add up when done across the whole trial system.
Trial Forge will make trials more efficient by looking for marginal gains across all trial processes, from research question to implementation into routine care. It will encourage everyone connected with trials to be more sceptical of what we do by asking for the evidence behind all of our trial decisions.
Trial Forge will make trials more efficient by looking for marginal gains across all trial processes, from research question to implementation into routine care. It will encourage everyone connected with trials to be more sceptical of what we do by asking for the evidence behind all of our trial decisions.
While the research questions and ideas can be novel and ambitious, some of the more challenging questions can often be found in teasing out the methodological issues that surround a clinical trial.
Dr. Sandra GalvinBSc PhD - Coordinator - Health Research Board - Trials Methodology Research Network
In the context of clinical trials, ‘‘quality’’ can be generally defined as the absence of errors that matter to decision-making—that is, errors which have a meaningful impact on the safety of trial participants or the credibility of the results (and thereby the care of future patients). (Clinical Trials. 2016 Apr 20.)
Ann Meeker-O’Connell & ColleaguesPart of the Clinical Trials Transformation Initiative
Researchers research so that we as patients and our clinicians can have better conversations
Derek StewartAssociate Director for Patient & Public Involvement & Engagement with the National Institute for Health Research
If research was a transport business, we would be appalled by these data. Half the goods carried would be badly designed, half lost in shipping, and half of the remainder broken by the time they arrived—a truly heart breaking waste.
Paul Glasziou And Iain ChalmersLead (PG) and founder member (IC) of the REWARD Alliance
Supposing is good, but finding out is better.
Mark Twain American writer (November 30, 1835 – April 21, 1910)
There is a peculiar paradox that exists in trial execution - we perform clinical trials to generate evidence to improve patient outcomes; however, we conduct clinical trials like anecdotal medicine. (Heart Fail Review 2012; 19: 135-52)
Monica ShahNational Heart Blood and Lung Institute, Bethesda, USA.
There are a lot of observational studies on recruitment. Are they useful? Hard to say, though I aim to find out. If they turn out to provide little to guide recruitment decisions, we should stop doing them, or they effectively become a recurring example of research waste.
Heidi GardnerResearch Assistant, University of Aberdeen
LATEST NEWS & EVENTS
A list of 11 priority recruitment and retention SWATs
Studies Within A Trial (SWATs) are a good way of generating evidence to support trial process decision-making. SWATs are designing to be replicated, which means it's important to have some focus otherwise we end up [...]
Resources to help with the design and delivery of SWATs
The Trial Forge SWAT Centre (University of York) in conjunction with the Trial Forge SWAT Network have recently been working to develop resources on the design and delivery of Studies Within A Trial (SWATs). These [...]
LATEST RESOURCES
Trial Forge Guidance 4: a guideline for reporting the results of randomised Studies Within A Trial (SWATs)
Trial Forge Guidance 4 helps trial teams to develop SWAT reporting guideline will aid authors, reviewers, and journal editors to
- Resource Type: Guidance
- Date Posted:
Principles for handling end-of-participation events in trials (PeRSEVERE)
The right to stop taking part, or change how to take part PeRSEVERE has its own website: https://persevereprinciples.org/ All guidelines,
- Resource Type: Guidance, Resource collection
- Date Posted:
test page
- Date Posted: